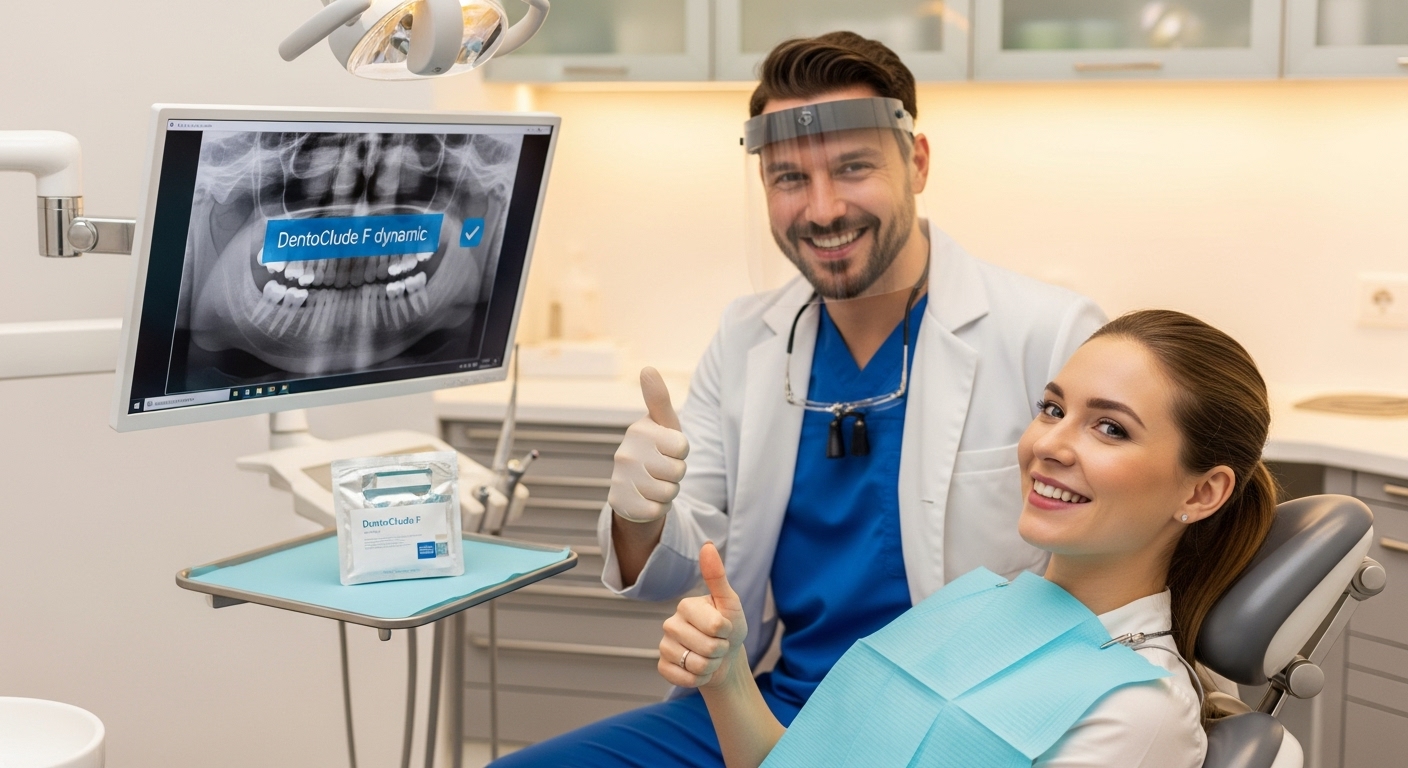
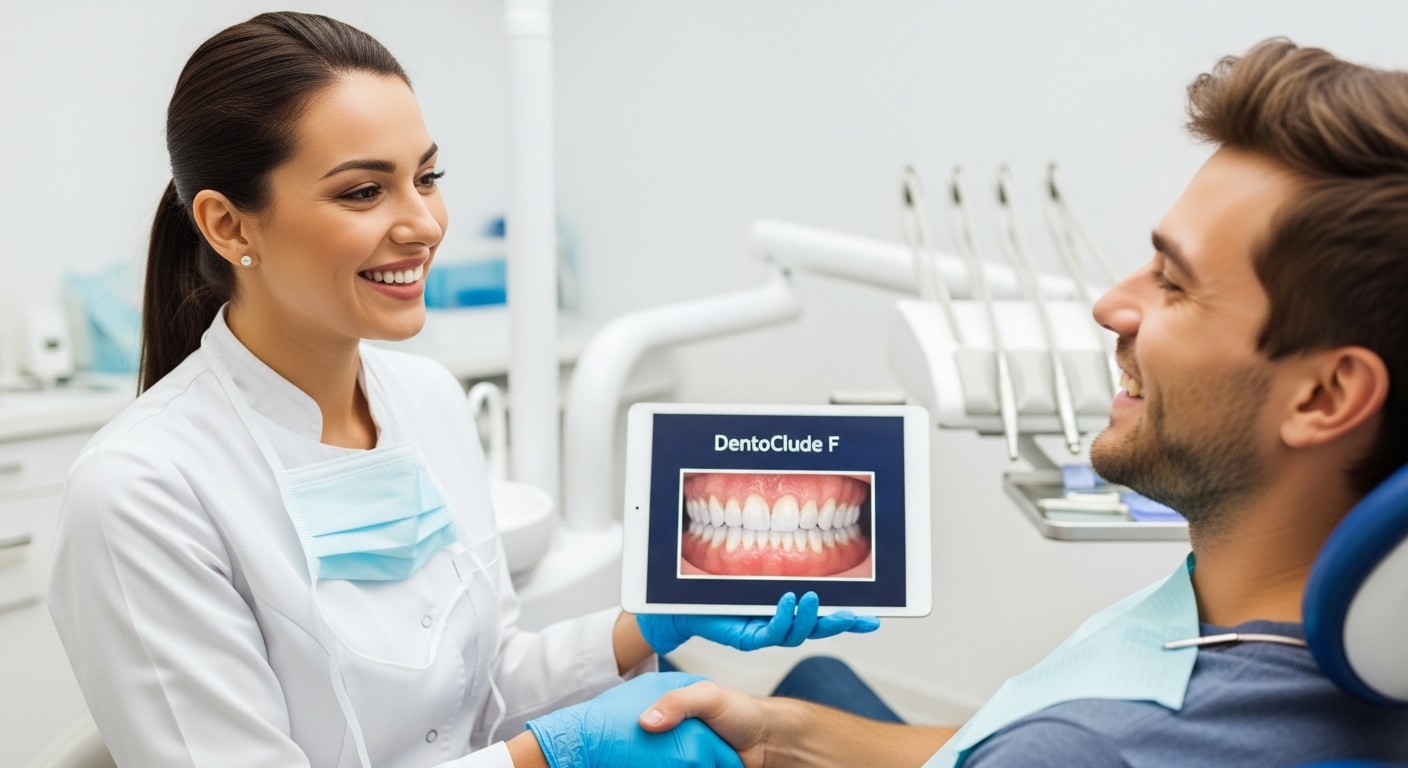
DentoClude F is an in‑office, FDA‑cleared desensitizing device Gel
Fast relief, lasting protection.
First in Class: DentoClude F is a bioactive glass technology used to treat and improve oral health. This technology works in the tooth restoration process to help prevent sensitivity caused by micro-leakage in dentinal tubles.
Blocks Heat & Cold Sensitivity
Advanced bioactive glass formula creates a protective barrier that shields dentin tubules from temperature changes, providing immediate and lasting relief from sensitivity.
Superior 3X Bonding Strength
Clinically proven to provide three times stronger bond strength compared to traditional desensitizing agents, ensuring restorations last longer.
Prolongs Restoration Life
Forms a durable protective layer that prevents micro-leakage and extends the longevity of dental restorations, saving costs in the long run.
Instant Relief
Patients experience immediate sensitivity relief following application, with effects lasting for months after a single treatment
Biocompatible Formula
Made with submicron bioactive glass, fluoride, and natural botanical extracts that are safe and promote tissue healing
Quick Application
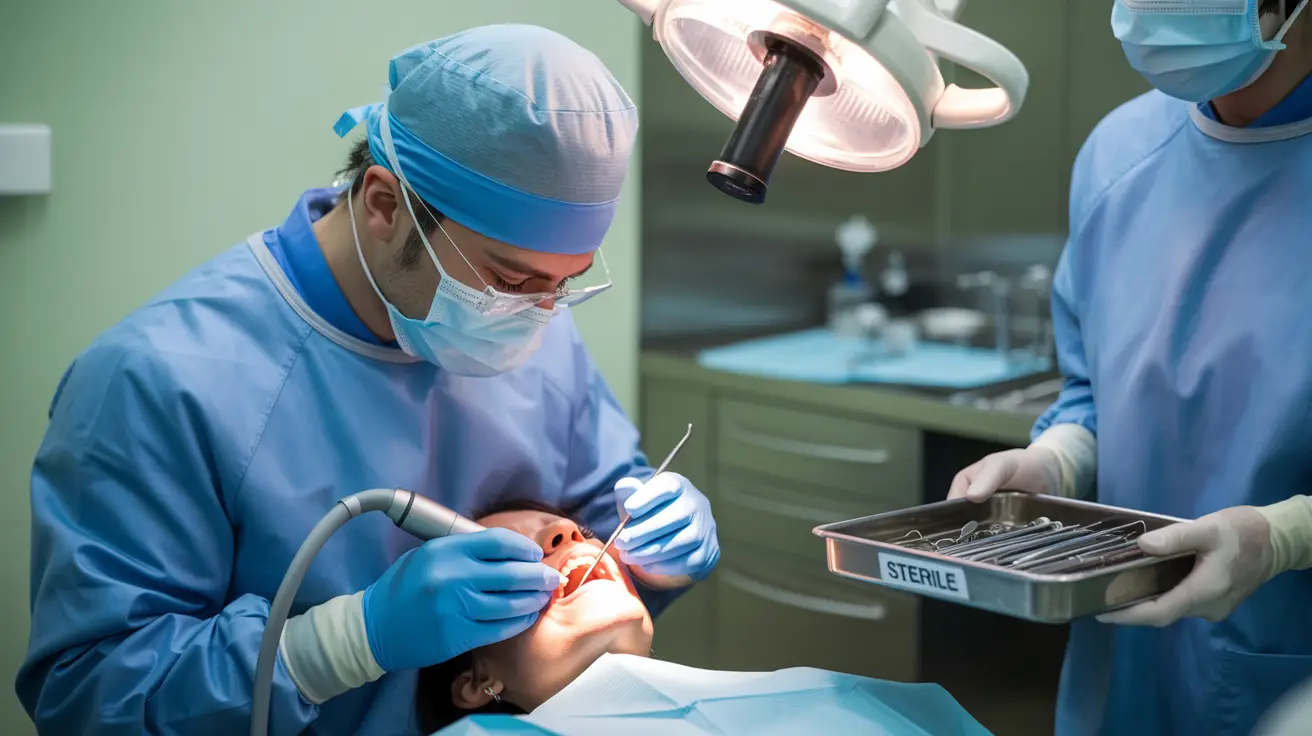
Simple chairside procedure that takes just minutes to apply, integrating seamlessly into any dental practice workflow
Clinically Validated
Practice ROI
Scalable Growth

DENTOCLUDE F
Practice Solutions
DentoClude™ F with fluoride is intended to be used in the manner based on indications for use. DentoClude™ F contains Bioactive Glass with particle size of less than 1μm as the key ingredient designed to fill and block the dentin tubules. DentoClude™ F Tubule Agent should be used during the restoration of teeth. DentoClude™ F is applied to the exposed dentin and the solvent evaporated off, leaving the bioactive glass. DentoClude™ F is manufactured in such a way that it is intended to enter the exposed dentin tubules and penetrate to a greater depth that prevents microleakage and sensitivity. In addition, Bioactive Glass in studies suggests having an anti-inflammatory effect
You ask, we answer
DentoClude F is an in‑office, FDA‑cleared desensitizing device gel that blocks dentin tubules, reduces micro‑leakage, and strengthens bonds, giving practices both a clear patient‑care benefit and a strong economic upside. Available is a brochure for dentists and technicians, emphasizing clinical use and ROI.
DentoClude F consistently produces statistically significant reductions in VAS and facial rating scale pain scores from baseline through 4–6 weeks, with superior improvements versus GLUMA, stannous fluoride gel, Sensolin, and other bioactive‑glass agents in head‑to‑head studies.
DentoClude F is a special dental gel that dentists apply to sensitive teeth to help stop pain from hot, cold, or sweet foods. It uses a tiny glass-like mineral (called bioactive glass with fluoride and natural plant extracts) that flows into the open channels in the tooth and gently “plugs” them, so sensations cannot easily reach the nerve. Over time, this material helps the tooth rebuild a natural, protective layer, giving longer-lasting relief than many regular sensitivity products you buy at the store. It is approved for use by regulators in the United States and India and is only available through licensed dental professionals, so patients receive it as part of an in-office treatment rather than as a home product. REQUEST INFO
“DentoClude F represents the kind of innovation investors dream of being early to—where sound science meets human transformation. Each restored smile isn’t just dentistry; it’s validation that regeneration is possible. This is not a product chasing a market—it’s a movement redefining what dental restoration means. Early investors aren’t simply funding growth; they’re shaping the standard of oral health for decades to come.” RECEIVE INVESTOR INFO
DentoClude F is an in‑office, FDA‑cleared desensitizing device that blocks dentin tubules, reduces micro‑leakage, and strengthens bonds, giving practices both a clear patient‑care benefit and a strong economic upside. Below is brochure‑style copy you can drop into a 1–2 page Handout or trifold, aimed at dentists and technicians, emphasizing clinical use and ROI. DentoClude™ F is now offering a limited complimentary practice program that lets your team trial the in‑office gel, receive onboarding, and integrate it into existing sensitivity protocols with no upfront product cost for qualifying offices. CLICK FOR ASSISTANCE
What is clearly included
Complimentary units of DentoClude F for chairside use so the team can treat real cases during the evaluation period.
Clinical onboarding, which typically means training and guidance on indications, chairside protocol, and how to integrate the gel into hygiene, restorative, and periodontal workflows.
Chairside integration support, i.e., help for the practice team to incorporate DentoClude F into their existing sensitivity management and post‑treatment protocols
The DentoClude F usage video provides clear, step-by-step instructions for dental professionals administering the product to patients experiencing tooth sensitivity and pain. It demonstrates proper application techniques, including opening the DentoClude F packaging, using an applicator to dispense the product, and precisely applying it to targeted tooth surfaces—especially those exhibiting hypersensitivity. The video emphasizes patient comfort, clean application, and following all safety and hygiene protocols to ensure optimal product performance.
DentoClude™ F with fluoride is intended to be used in the manner based on indications for use. DentoClude™ F contains Bioactive Glass with particle size of less than 1μm as the key ingredient designed to fill and block the dentin tubules. DentoClude™ F Tubule Agent should be used during the restoration of teeth. DentoClude™ F is applied to the exposed dentin and the solvent evaporated off, leaving the bioactive glass. DentoClude™ F is manufactured in such a way that it is intended to enter the exposed dentin tubules and penetrate to a greater depth that prevents microleakage and sensitivity. In addition, Bioactive Glass in studies suggests having an anti-inflammatory effect.
An interactive map of practice locations will be available 2/1/2026. Please ask your dentist today!
Dentoclude F uses submicron bioactive glass particles that penetrate 5-10μm into dentin tubules. When exposed to oral fluids containing calcium and phosphate, these particles precipitate hydroxyapatite, physically occluding the tubules and creating a durable barrier against external stimuli like hot, cold, and sweet foods.
Yes, Dentoclude F can be used with self-etch adhesives. Apply the desensitizer immediately after the self-etch adhesive. The acidic monomers in the adhesive dissolve the smear layer and demineralize dentin, allowing both the bioactive glass and adhesive to penetrate and form a strong hybrid layer.
No, in fact Dentoclude F has been shown to improve bond strength. Clinical studies demonstrate that bioactive glass can increase bond strength by up to 3X compared to control groups. The material integrates into the adhesive layer without compromising mechanical properties.
The effects of Dentoclude F are long-lasting. Clinical studies have shown sustained sensitivity relief for 3 months and longer after a single application. The bioactive glass continues to interact with oral fluids, maintaining the protective hydroxyapatite layer over time.
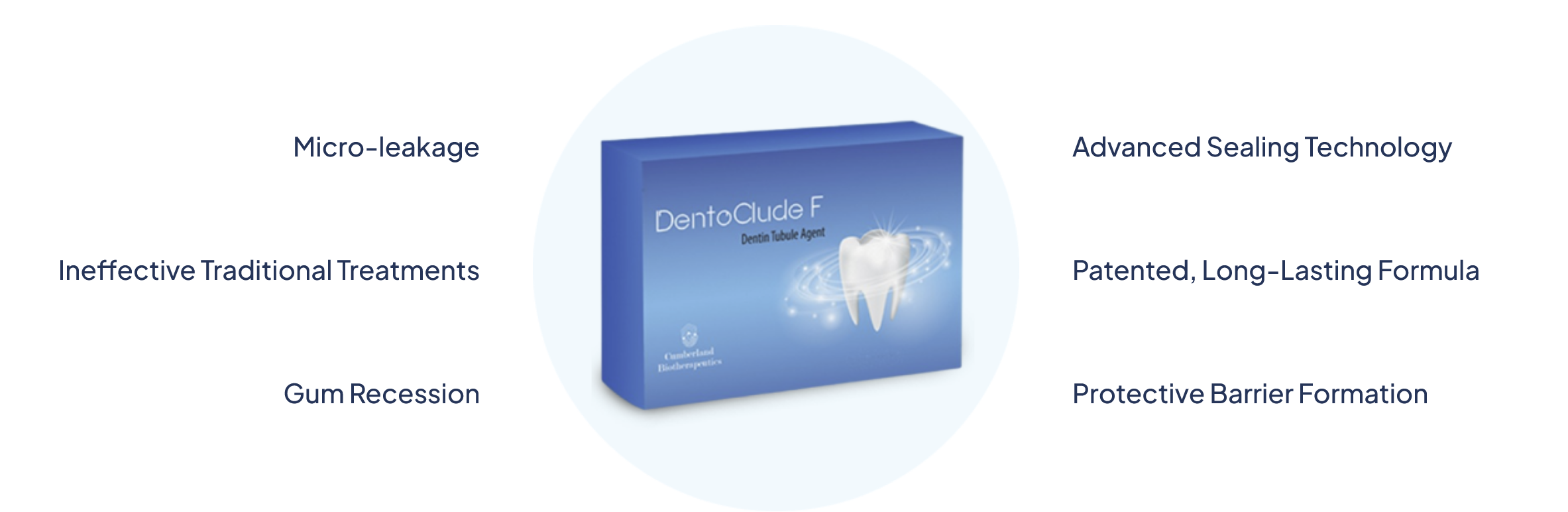


Cumberland Biotherapeutics developed DentoClude F® technology with bioactive glass with Fluoride, particle size of <1 µm that contains botanicals with anti-infective & anti-inflammatory active extracts. DentoClude F® is approved by the US FDA, USA and DCGI/ AYUSH, India. DentoClude F® is available only through your Dental Provider. DentoClude F®, a NOVEL device with Bioactive Glass that blocks dentin tubules and prevents micro-leakage resulting in a decrease in sensitivity at root surface exposed due to periodontal disease or post periodontal therapy as well as periodontal and implant bone regeneration.

Hypersensitivity
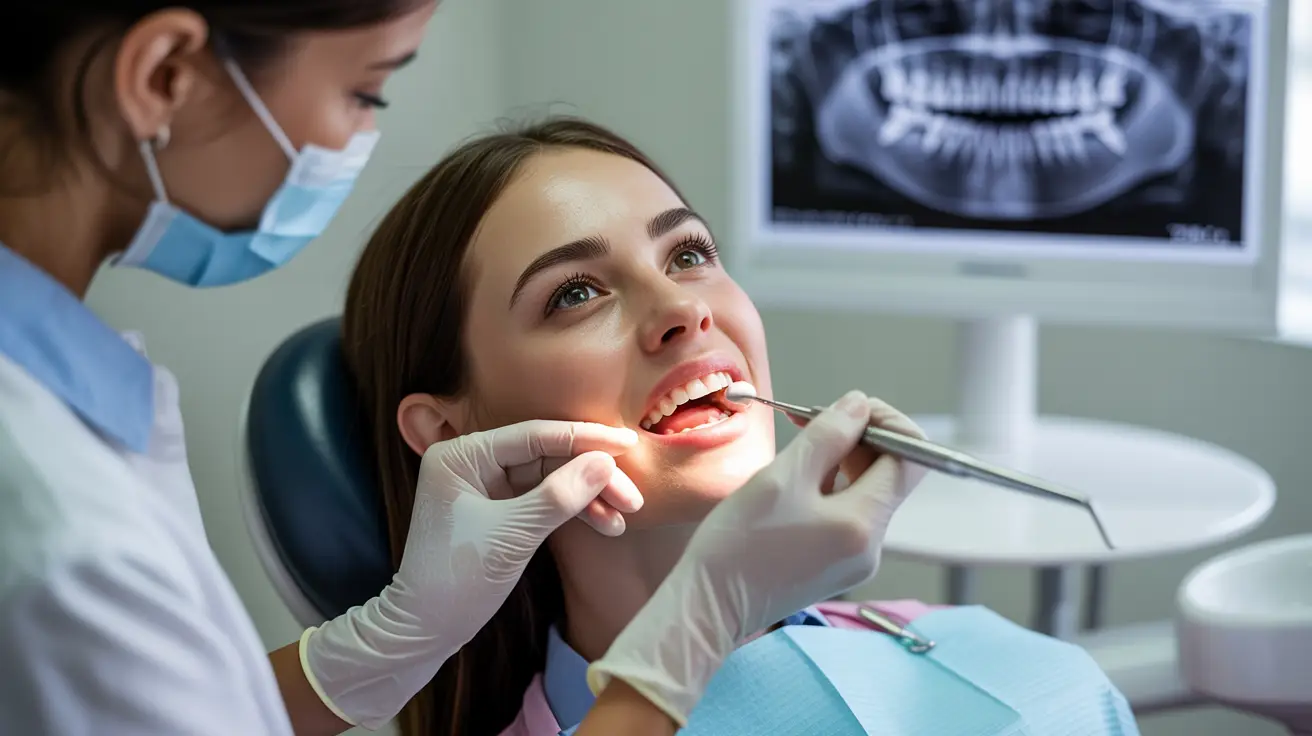
Study Information

DentoClude F Comp Program

Investment Information

Patient Questions

Doctor Podcast

DentoClude F Business
Critical Data
DentoClude F studies report very high satisfaction, with about 97% of patients willing to recommend treatment and dentists citing easier eating and drinking, less discomfort, and favorable handling after a single session.
Available summaries state that DentoClude F has demonstrated superior efficacy to Sensodyne in comparative clinical contexts, offering more rapid and longer‑lasting sensitivity relief by permanently sealing dentinal tubules after one in‑office application.
Simple DentoClude F Explanation

Order your complimentary DentoClude F units today
2026
Contact Us
Reach us through
-
USA HQ
1565 Jackson Point Road, Sewanee, Tennessee 37375
INDIA HQ
70 to 73, Ground Floor, EVK Complex, Krishna Nagar Cross Road Junction, Jubilee Hills, Hyderabad-500045, India - +1-800-845-0279
- solutions@dentocludef.net
Social Networks
- Cumberlandtx
- cumberlandtx
- cumberlandtx
- Cumberlandtx
Send us a Message or Request Units
© 2025 Cumberland Bio All Rights Reserved.


